UROADVANCE: An advanced non-invasive genetic test for detection of tumor DNA mutations in urine related with bladder cancer
GENOMICA is proud to introduce you UROADVANCE, an innovative and advanced genetic test that allows the non-invasive (through the analysis of a urine sample) detection of somatic mutations of urinary tumor DNA (utDNA) deriving from urothelial cells, associated with bladder cancer. The UROADVANCE test allows for early identification of bladder cancer, significantly increasing the chances of therapeutic success.
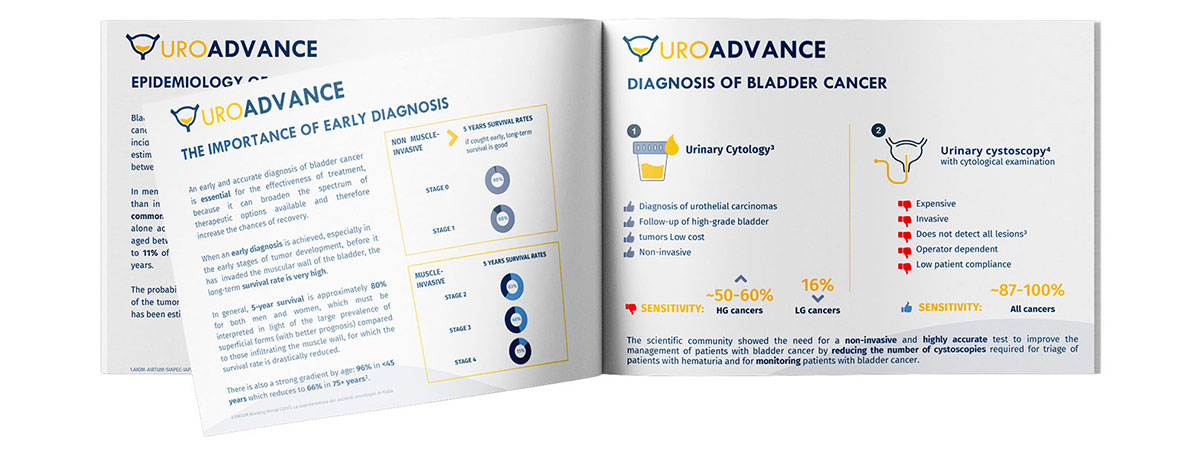
Bladder cancer represents approximately 3% of all cancers, affecting over 313,500 individuals. An incidence of around 30,000 cases/year is estimated, with onset prevalent in old age, between 60 and 70 years old.
An early and accurate diagnosis of bladder cancer is essential for the effectiveness of treatment, because it can broaden the spectrum of therapeutic options available and therefore increase the chances of recovery. When an early diagnosis is achieved, especially in the early stages of tumor development, before it has invaded the muscular wall of the bladder, the long-term survival rate is very high (~80%).
The UROADVANCE test uses the latest technological innovations developed for liquid biopsy. Thanks to the state-of-the-art DNA sequencing technology, named Next Generation Sequencing (NGS), it is possible to effectively identify, through a simple and non-invasive urine sample, somatic mutations of urinary tumor DNA in 22 genes commonly mutated in bladder tumors.
UROADVANCE is able to detect somatic mutations present in minimal percentages (up to 0.5% of Mutant Allele Frequency - MAF), and therefore even in the presence of small quantities of tumor cells.
It is a highly sensitive test, equivalent to cystoscopy, for all grades and stages of bladder cancer, including non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC);
It has the potential to be used across the entire bladder cancer therapeutic treatment pathway, including patient monitoring, surveillance for recurrence and minimal residual disease (MRD), as well as haematuria triage.
It uses a simple urine sample to detect somatic mutations in urinary tumor DNA (utDNA) derived from urothelial cells
Further information on the UROADVANCE test can be obtained through the website UROADVANCE.IT or by requesting the assistance of our team of geneticists.
If you are interested to receive a more information on UROADVANCE, we invite you to write to us
Contact Genomica
Stay tuned, to learn about the most advanced and innovative diagnostic solutions in the genetics field.
GENOMICA srl
Marketing and Communication Department